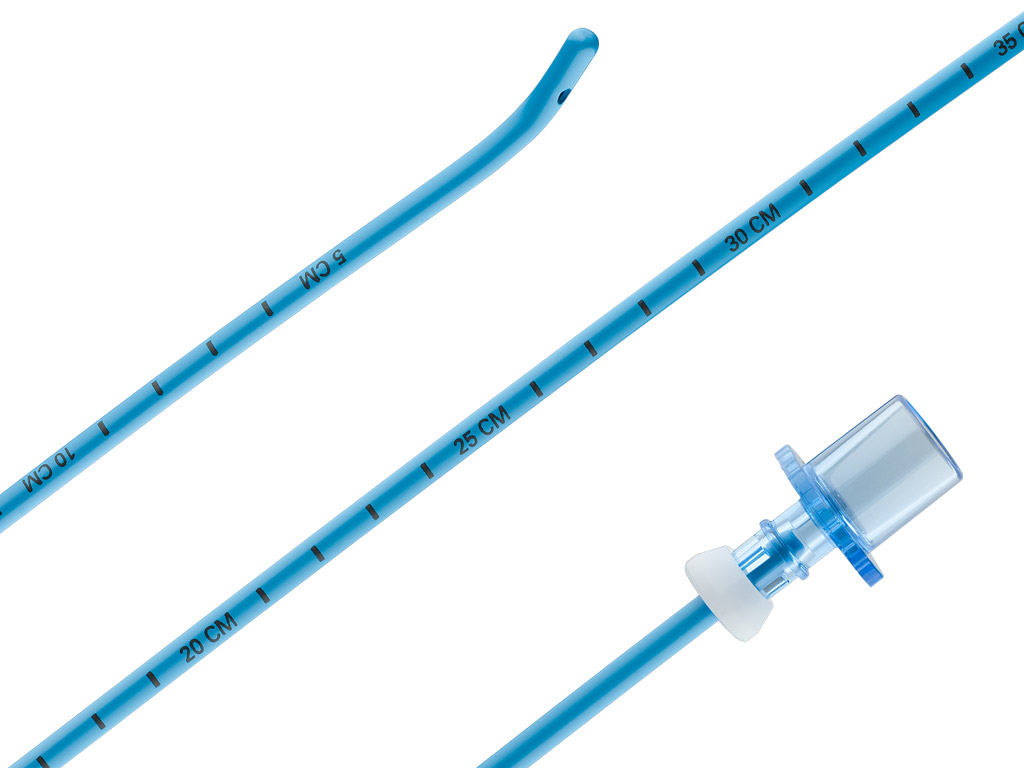
Frova Intubating Introducer
Intended to facilitate endotracheal intubation in patients where the visualization of the glottis is inadequate.
Features & Benefits
- The blunt, angled tip is designed to help you properly place the introducer beyond the vocal cords and into the trachea.
- The optional stiffening stylet1 adds rigidity to the proximal and middle sections of the catheter, while the distal portion remains flexible.
- The lumen and sideports are channels by which oxygen may be delivered when the device is used with Rapi-Fit® adapters and an oxygen source.
- The centimeter marks on the catheter facilitate accurate alignment with endotracheal tubes.
- The removable Rapi-Fit adapters permit oxygen delivery, if necessary.
- May also be referred to as a ‘cannula’.
See full image.

Find ordering information and other resources for this product at cookmedical.com.
ESSENTIAL PRESCRIBING INFORMATION
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).
INTENDED USE: The Frova Intubating Introducer is intended to facilitate endotracheal intubation in patients where the visualisation of the glottis is inadequate. The 14 French catheter introducer has been designed for placement of a single lumen endotracheal tube whose inner diameter is 6 mm or larger.
CONTRAINDICATIONS: Do not use if the epiglottis cannot be visualised when performing laryngoscopy, i.e, Grade IV Cormack & Lehane laryngoscopy classification.
WARNINGS: Do not use the Frova Intubating Introducer with double lumen endotracheal or endobronchial tubes. • To avoid barotrauma and/or pneumothorax, examine the patient’s anatomy to help determine the optimal placement for the Frova Intubating Introducer. Ensure the catheter is in the physician-preferred location relative to the carina by referencing its centimeter markings. • Care must be taken not to provoke injury to the epiglottis and glottis, perforation of the sinus pyriformis, trachea or bronchus. • The use of removable Rapi-Fit Adapters permits the utilisation of a high- and low-pressure oxygen source, if necessary, during the procedure. Use of the Rapi-Fit Adapter for oxygenation may be associated with a risk of barotrauma. • Use of an oxygen source should only be considered if the patient has sufficient egression of the insufflated gas volume. • If a high-pressure oxygen source is used for insufflation (e.g., jet ventilator), begin at a lower pressure and work up gradually. Rising chest wall, pulse oximetry and oral air flow should be carefully monitored. Table 2 provides information on catheter oxygenation. • Ensure that the Rapi-Fit Adapter is securely connected to the Frova catheter prior to oxygen delivery. Failure to properly secure the adapter to the catheter introducer may result in hypoxia, hypoxaemia and serious adverse events. • Lubricate the catheter introducer and endotracheal tube before use. • Ensure proper sizing of the endotracheal tube to be used in combination with the Frova Intubating Introducer. • Care must be taken when introducing/removing the catheter introducer from the endotracheal tube; contact with sharp edges on the internal surface of the endotracheal tube may cause small fragments to be shaved off the catheter introducer during introduction/removal. • Possible allergic reactions (e.g., to butyl rubber) should be considered.
PRECAUTIONS: The product is intended for use by physicians trained and experienced in airway management. • Sizing of the Rapi-Fit Adapter and the Frova Intubating Introducer must match in order to provide oxygenation through the catheter introducer. • Not for intravascular use.
POTENTIAL ADVERSE EVENTS: Allergic reaction (e.g., to butyl rubber) • Barotrauma • Hypoxia or hypoxaemia • Injury to the epiglottis and glottis, perforation of the sinus pyriformis, trachea or bronchus • Pneumothorax
See Instructions for Use for full product information.
AB_I-CAE-FII-239-16_REV0
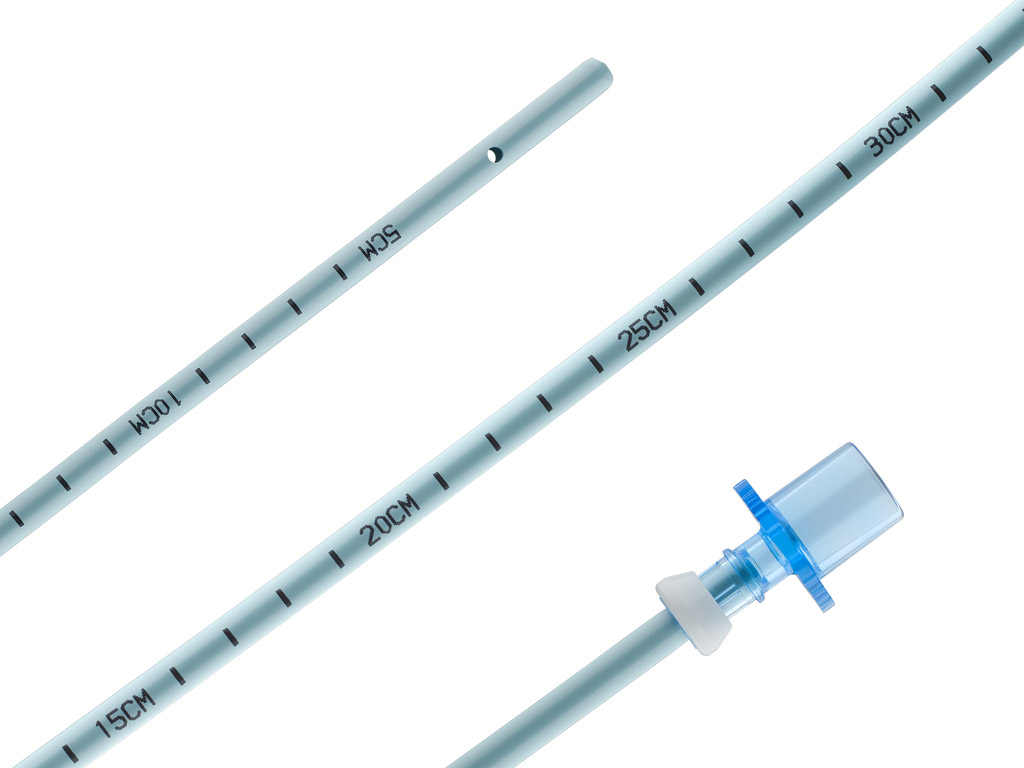
Aintree Intubation Catheter
Intended for assisted fiberoptic intubation and for uncomplicated, atraumatic endotracheal tube exchange.
Features & Benefits
- The catheter’s inner diameter (ID), lumen, and distal sideports are designed to enhance airflow.
- The large ID permits the use of a fibreoptic scope with a maximum outer diameter of 4.2 mm.
- The centimeter marks facilitate accurate alignment with endotracheal tubes.
- The removable Rapi-Fit adapters permit oxygen delivery, if necessary.
See full image.
Find ordering information and other resources for this product at cookmedical.com.
ESSENTIAL PRESCRIBING INFORMATION
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).
INTENDED USE: The Aintree Intubation Catheter has been designed for assisted fiberoptic intubation and for uncomplicated, atraumatic endotracheal tube exchange.
CONTRAINDICATIONS: None known
WARNINGS: Possible allergic reactions should be considered. • Do not advance the catheter beyond the carina. Attention should be paid to the depth of insertion of the catheter into the patient’s airway, and to the correct tracheal position of the endotracheal tube. Markers on the catheter refer to distance from the tip of the catheter. • Take care not to injure the epiglottis and glottis, or to perforate the sinus pyriformis, trachea or bronchus. • Use of the Rapi-Fit adapter for oxygenation may be associated with a risk of barotrauma. • Use of an oxygen source should only be considered if the patient has sufficient egression of the insufflated gas volume. • If a high-pressure oxygen source is used for insufflation (e.g., jet ventilator), begin at a lower pressure and work up gradually. Rising chest wall, pulse oximetry and oral air flow should be carefully monitored. • Ensure proper sizing of the catheter within an endotracheal tube. Failure to do so may cause small fragments to be shaved off during removal of the catheter. • To avoid barotrauma, ensure that the tip of the AIC catheter is always above the carina, preferably 2-3 cm.
PRECAUTIONS: This product is intended for use by physicians trained and experienced in airway management techniques. Standard techniques for placement and exchange of endotracheal tubes should be employed. • This product is not intended for intravascular use. • Lubricate the catheter and endotracheal tube before use. • Do not attempt to place a fiberoptic scope through the Rapi-Fit adapter. The adapter must be removed prior to scope insertion. • The potential effects of phthalates on pregnant//nursing women or children have not been fully characterized and there may be concern for reproductive and developmental effects.
POTENTIAL ADVERSE EVENTS: Barotrauma • Perforation of the bronchi or lung parenchyma • Pneumothorax
See Instructions for Use for full product information.
AB_C_T_AIC_REV1
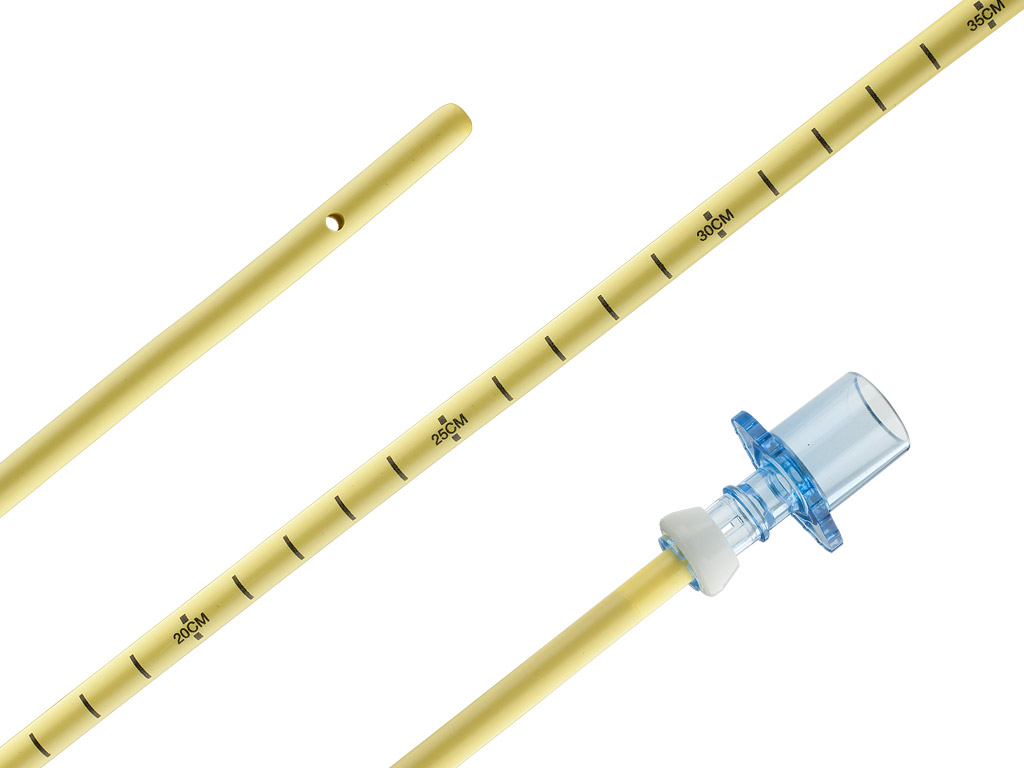
Cook® Retrograde Intubation Set
The Cook Retrograde Intubation Set is intended to assist in intubation during difficult airway access procedures in adult and pediatric patients.1
Features & Benefits
- Using the Seldinger technique via the cricothyroid membrane for initial access permits retrograde (cephalad) placement of a wire guide that exits orally or nasally.
- The catheter’s tapered tip allows for smoother navigation through the patient’s anatomy.
- The antegrade introduction of a hollow intubation catheter with distal sideports and Rapi-Fit adapters allows you to provide the patient with oxygen and facilitates the placement of an endotracheal tube.
- The 6 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 2.5 mm or larger. The 11 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 4.0 mm or larger. The 14 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 5.0 mm or larger. When used for high-pressure oxygenation with a Luer lock connector, the 14 French intubation catheter is recommended for patients older than 12 years of age.
For complete instructions, age-specific recommendations, contraindications, warnings, and precautions, see the Instructions for Use included with each product.
See full image.

Find ordering information and other resources for this product at cookmedical.com.
ESSENTIAL PRESCRIBING INFORMATION
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).
INTENDED USE: The Cook Retrograde Intubation Set is intended to assist in intubation during difficult airway access procedures in adult and pediatric patients. The 6 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 2.5 mm or larger. The 11 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 4.0 mm or larger. The 14 French intubation catheter is recommended for placement of an endotracheal tube with an inner diameter of 5.0 mm or larger. When used for high-pressure oxygenation with a Luer lock connector, the 14 French intubation catheter is recommended for patients older than 12 years of age.
CONTRAINDICATIONS: Coagulopathy • Laryngotracheal disease • Obscure cricothyroid anatomy • Infection of cricothyroid membrane • Mass (i.e., goiter) • Lack of anatomical markers • Impossible access to the airway
WARNINGS: Clinicians should first select the appropriate sized endotracheal tube (ETT) based on individual patient anatomy, weight, and recommendations from the manufacturer of the ETT. The appropriate sized intubation catheter for the retrograde intubation procedure may then be determined based on the chosen ETT. • Do not advance the intubation catheter beyond the carina. • Attention should be paid to the insertion depth of the intubation catheter into the patient’s airway and correct tracheal position of replacement endotracheal tube (ETT). Markers on the intubation catheter refer to distance from the distal tip of intubation catheter. • Take care to avoid injuring the epiglottis and glottis, and to avoid perforating the sinus pyriformis, trachea, or bronchus while using this device. For the 14.0 Fr Retrograde Intubation Catheter: Use of the Rapi-Fit adapter for oxygenation may be associated with a risk of barotrauma. • To avoid barotrauma, ensure that the tip of the intubation catheter is always above the carina, preferably 2-3 cm. • Ensure proper sizing of the intubation catheter within an ETT. • Use of an oxygen source should be considered only if the patient has sufficient egression of the insufflated gas volume. • Oxygen insufflation may not be appropriate for all patient subgroups and with all products. Please refer to the Catheter Oxygenation Table for product and patient subgroup details. • If a high-pressure oxygen source is used for insufflation (e.g., jet ventilator), begin at a lower pressure (i.e., 5 psi) and work up gradually. Rising chest wall, pulse oximetry, and oral air flow should be carefully monitored. • Ensure that the Rapi-Fit Adapter is securely connected to the intubation catheter prior to oxygen delivery. Failure to properly secure the adapter to the intubation catheter may result in hypoxia and serious adverse events. • High pressure oxygenation with Luer lock connector should only be used in patients older than 12 years old. If used in patients 12 years old or younger, the maximum airway pressure may be higher than 28 cm H20.
PRECAUTIONS: This product is intended for use by clinicians trained and experienced in retrograde intubation techniques. This device is limited to use in hospitals, surgical centers, and acute care centers. Standard techniques for retrograde intubation should be employed. • Patients in need of retrograde intubation may have significant spinal injury. In patients who have sustained significant trauma, the cervical spine should be immobilized throughout the procedure, if possible. • Whenever possible and appropriate, utilize aseptic technique and local anesthetic for the procedure.
POTENTIAL ADVERSE EVENTS: Barotrauma • Esophageal perforation • Airway bleeding • Pneumomediastinum • Pneumothorax • Hypoxia • Subcutaneous emphysema • Infection • Hematoma • Catheter dislodgment or migration • Wire guide dislodgment or migration • Failed endotracheal tube placement
See Instructions for Use for full product information.
AB_C_T_RETRO2_REV0